Chimeric antigen receptor (CAR) T cells are remarkably effective, especially against B-cell malignancies. However, CAR-T cell therapy is also associated with cytokine-driven inflammatory toxicities such as cytokine release syndrome. Similar cytokine-related toxicity can occur after bispecific T-cell engager (BiTE) administration. It is unknown whether these inflammatory responses are specifically related to T-cell-mediated therapies that are activated through artificial CAR or BiTE signals.
Cytokine-driven inflammatory toxicities were not commonly encountered prior to the wide-spread use of CAR-T cells in the clinic–neither from the adoptive transfer of ex vivo-expanded autologous polyclonal antigen-specific T cells, tumor-infiltrating lymphocytes, nor TCR-engineered T cells (TCR-T cells). However, it is challenging to compare these diverse cell products, utilized in different clinical contexts and against different antigens. Preclinical comparisons of CARs and TCRs consistently showed lower sensitivity of CARs for antigen as compared to that of TCRs, at least partly due to inefficient downstream signaling of the former (Salter AI et al. Science Signaling. 2021; Gudipati V et al. Nat Immunol. 2020; Harris DT et al. J Immunol. 2018, Oren R et al. J Immunol. 2014). Nonetheless, it is not known whether these differences in signaling and T cell effector functions affect the preponderance of eliciting inflammatory responses.
Here, we discovered a TCR targeting the CD22 antigen expressed by various B-cell malignancies in order to evaluate whether anti-leukemia/lymphoma T-cell cytotoxicity could be achieved without causing inflammatory responses by using TCR-activated T cells rather than CAR-activated T cells for the same antigen, CD22. A TCR specific to HLA-A*02:01-restricted epitope of human CD22 was isolated from an HLA-A2 negative healthy donor T cells through allogeneic in vitro stimulations. TCR alpha/beta-paired single cell sequencing was performed, and the sequence of the most enriched TCR clonotype was reconstructed in a retroviral expression vector. Primary human T cells transduced with this CD22 TCR recognized malignant B-cell lines only when they expressed both HLA-A*02:01 and CD22, confirming the intended specificity of the TCR. The TCR did not cross-react with other human proteins.
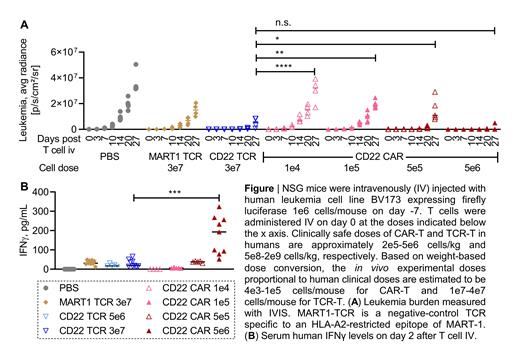
CD22 TCR-T cells were then compared with CD22 CAR-T cells that are successful in the treatment of patients with CD22+ leukemia/lymphoma but associated with dose-limiting inflammatory toxicities (Shah NN et al. J Clin Oncol. 2020, Frank MJ et al. Blood. 2021). CD22 TCR-T cells mediated efficient in vitro cytotoxicity against CD22-dim lymphoma cells whereas CD22 CAR-T cells exerted suboptimal cytotoxicity. Importantly, TCR-T cells (1e7-3e7 cells/mouse) also cleared leukemia in xenograft models without inducing systemic proinflammatory cytokine elevation (Figure). In contrast, CD22 CAR-T cells, given at the dose necessary to control in vivo leukemia growth (5e6 cells/mouse), led to significantly increased levels of circulating proinflammatory cytokines such as IFNγ (Figure), TNFα, GM-CSF, MIP-1α and MIP-1β. Systemic cytokine elevation after CAR-T cell infusion exacerbated when NSG xenograft mice were pre-infused (24 hours prior to adoptive T cell transfer) with PBMC containing myeloid cell populations that were autologous to T cells. TCR-T cell therapy was not associated with proinflammatory cytokine elevation even in the presence of myeloid cell populations. Consistent with these in vivo observations, in vitro cytokine production by CAR-T cells were higher compared to TCR-T cells in response to CD22+ leukemia cells. Moreover, T cells activated through the CD22 TCR and CD22 CAR by the identical leukemia cell line demonstrated differential transcriptional signatures; CAR-T cells exhibited a disproportionate and significant upregulation of inflammatory pathways compared to TCR-T cells while effector response pathways were either equivalent or enriched in TCR-T cells. Together, these results highlight the opportunity for TCR-T cells to mediate anti-leukemia responses with a potentially safer toxicity profile compared to CAR-T cells. Our work provides the basis for incorporating TCR-based immunotherapy into the treatment paradigm for patients with relapsed/refractory B-cell malignancies, improving the safety of adoptive cell therapy.
Disclosures
Davies:Genentech: Current Employment. Kadakia:Precigen: Current Employment. Rae:10x Genomics: Current Employment. Hinrichs:PACT Pharma: Consultancy; GlaxoSmithKline: Consultancy; Neogene Therapeutics: Consultancy, Research Funding; Capstan Therapeutics: Consultancy; T-Cure Biosciences: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal